SADA PRIT
Technology Platform
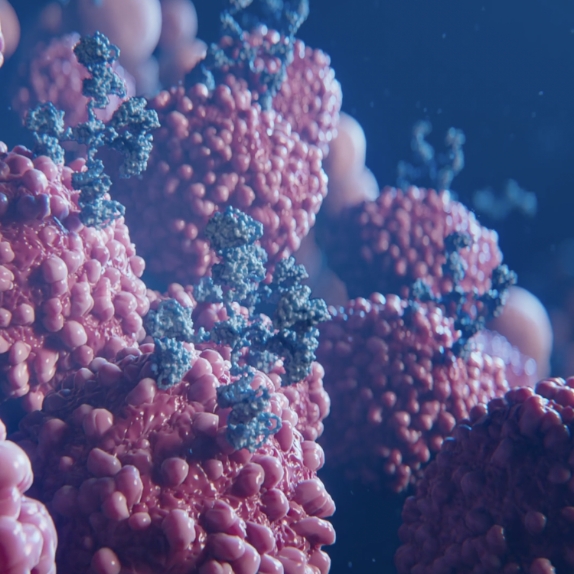
Y-mAbs’ SADA (Self-Assembly DisAssembly) PRIT (Pre-Targeted Radioimmunotherapy) Technology Platform
Our proprietary SADA PRIT Technology Platform is pharmacokinetically optimized and designed with the future treatment paradigm beyond just cancer. The platform is designed to pre-target tumors or disease cells with precision, enabling us to potentially open the therapeutic window from both sides – by minimizing off-target toxicity and by allowing highly focused
on-target radiation, which have been demonstrated pre-clinically to date.
We are currently conducting a first-in-human, multicenter Phase I trial that is actively enrolling at U.S. sites.
The SADA PRIT Technology Platform is investigational. Safety and efficacy have not yet been established nor approved by health authorities, and it is not an approved treatment for any disease.
Radiopharmaceuticals Pipeline
Asset
Target
Therapeautic Areas
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
SPONSOR
GD2-SADA-177Lu-Proteus
GD2
Recurrent/Refractory Small Cell Lung Cancer (SCLC), Sarcoma, Malignant Melanoma, and High-Risk Neuroblastoma
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
89Zr-DFO-naxitamab
GD2
GD2-Expressing Solid Tumors
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
CD38-SADA
CD38
Relapsed/Refractory Non-Hodgkin Lymphoma
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST01-PRIT
Undisclosed
Colorectal Cancer
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST01-Dx
Undisclosed
Colorectal Cancer
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST02-PRIT
Undisclosed
Prostate Cancer
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST03-PRIT
Undisclosed
Solid Tumors
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST03-Dx
Undisclosed
Solid Tumors
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST04-PRIT
Undisclosed
Solid Tumors
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST05-PRIT
Undisclosed
Solid Tumors
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
Y-ST06-PRIT
Undisclosed
Solid Tumors
DISCOVERY
PRECLINICAL
FiH IMAGING
FiH THERAPY
PHASE I/II
Y-mAbs
How we designed the SADA PRIT Technology Platform
Pre-targeting 2-step approach: Separating pre-targeting of the tumor from radioactive payload delivery.
SADA PRIT Technology Platform
Due to its modularity, the SADA PRIT Technology Platform can be easily adapted to most tumor antigens, tumor types, or drug delivery approaches to improve therapeutic indices and maximize the delivered dose
Self-Assembled Tetramer
(~200kDa)
Designed to bind tumors with high activity
DisAssembled Monomers
(~70kDa)
Designed to be cleared rapidly
SADA=Self-Assembling Dis-Assembling
PRIT=Pre-targeted Radioimmuntherapy
2-Step Approach
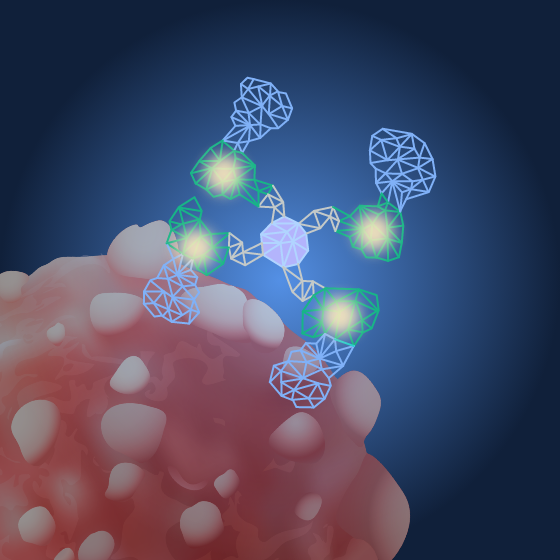
Step 1
SADA tetramers administered
SADA tetramers target and bind to tumors with high avidity
Unbound tetramers disassemble into SADA monomers and are excreted within a few days
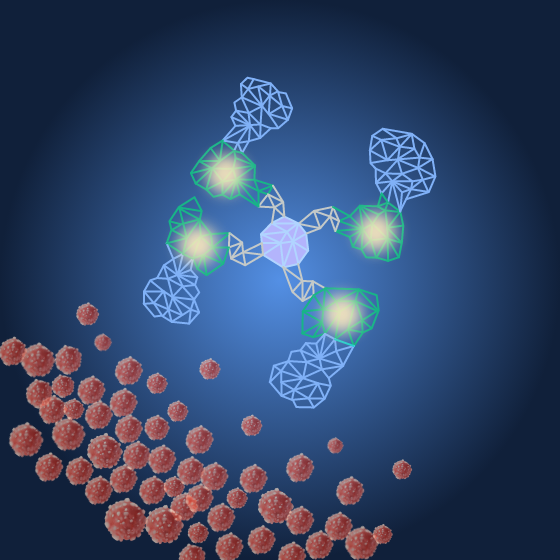
Step 2
Radioactive payloads administered after a few days
Radioactive isotope bind SADA tetramers already bound to tumors
• 177Lu-DOTA and 225Ac-Proteus
• 177Lu-DOTA used in first-in-human Phase 1 trial
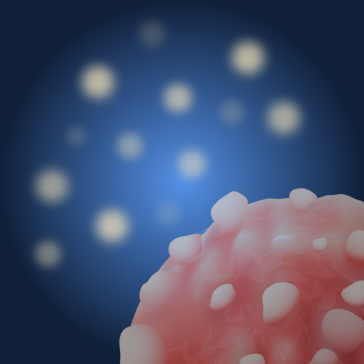
Unbound radioactive payloads are cleared within a few hours
Radioactive payloads irradiate tumors
Theranostic capability
Our SADA PRIT Technology Platform was designed to allow for pre-targeted imaging to ensure only patients with the confirmed tumor target who may benefit from treatment will receive a therapeutic radiation dose. Reduced off-target radiation exposure enables the therapeutic radiation dose to be highly focused to the tumor and potentially deliver higher radiation doses than what is currently possible.
Y-Labs: state-of-the-art laboratory
Y-Labs is our state-of-the-art laboratory, located in Nutley, New Jersey. Y-Labs has in vitro drug discovery, development, and characterization capabilities, allowing for hands-on experience with the early phases of each drug candidate. Our team has established streamlined processes and has already delivered submissions approved by the FDA.
The Y-Labs team can adapt our SADA PRIT Technology Platform, which was designed to be modular and flexible with interchangeable components with the goal of targeting a wide variety of tumors and deliver different radioactive payloads.
Ongoing SADA PRIT Technology Platform clinical development pipeline
Though we anticipate that our SADA PRIT Technology Platform will have applications across pediatric and adult oncology, and potentially beyond just cancer, our first-in-human clinical trial targets GD2 and focuses on patients with solid tumors, including small cell lung cancer, sarcoma, and malignant melanoma. This multicenter GD2-SADA Phase I trial is actively enrolling at US sites. Our second human clinical trial targets CD38 and focuses on patients with blood cancers, specifically Non-Hodgkin’s Lymphoma. We are currently in first-in-human, multicenter Phase I clinical trials and are actively enrolling at U.S. sites.
If you or someone you know has small cell lung cancer, sarcoma, malignant melanoma, or Non-Hodgkin’s Lymphoma and is interested in exploring participation in these studies and helping advance the development of this novel cancer platform, please click the link below to learn more about our clinical trials.
SADA PRIT Technology Platform is investigational. Safety and efficacy have not been established by health authorities and it is not an approved treatment for any disease.

